Polyacrylamides
Properties
Polyacylamides are high molecular weight water soluble or
swellable polymers formed from acrylamide or its derivatives. Their glass transition temperature is well above room temperature
(> 400 K).
The only commercially important polyacrylamide is poly(2-propenamide) which
is simply called polyacrylamide or PAM [-CH2CH(CONH2)-].
It is a non-ionic, watersoluble, and biocompatible polymer that can be tailored to meet
a broad range of applications. The polymer can be synthesized as a
simple linear chain or as a cross-linked structure. PAM increases
the viscosity of water and encourages the flocculation of particles
present in water. The cross-linked polymer can absorb and retain
extemely large amounts of water because the amide groups form strong
hydrogen bonds with water molecules. The hydrated PAM is a soft gel
that is used in gel electrophoresis and as a super water-absorbing
polymer (SAP's). Even though
these polymers are called polyacrylamide, they are often copolymers
of acrylamide and one or more other monomers. The most important
co-monomer is acrylic acid or sodiumacrylate. Poly(acrylamide-co-acrylic acid) and
its sodium salt are anionic polymers which are more effective as
flocculant and water absorber.
Very few other polyacrylamides have found industrial applications. Noteworthy are poly(N,N-dimethylacrylamide) (PDMA), poly(dimethyldiallylammonium chloride) (PDMDAAC), and poly(acrylooxyethyltrimethyl ammonium chloride) (PDAC) and copolymers thereof. PDMDAAC and PDAC are cationic polymers that can break emulsions and induce flocculation. As a primary organic coagulant, they neutralize negatively charged colloidal material and reduces sludge volume compared with inorganic coagulants. Poly(N,N-dimethylacrylamide) is useful as a chain transfer agent (CTA) and as a functionalized polymer. DMA is sometimes copolymerized with styrene and butadiene or with methylmethacrylate.
PAMs polymerize in the presence of free radicals. A typical initiator is ammonium persulfate and a typical stabilizer tetramethylethylenediamine. The most common crosslinker is bisacrylamide. The polymerization reaction creates a high molecular weight polymer or gel.
| Polyacrylamide | Structure of Repeat Unit |
| Anionic Polyacrylicamide (APAM) |
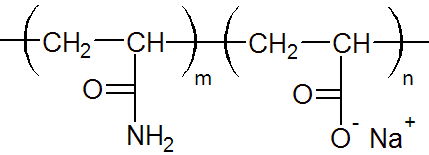
|
| Neutral Polyacrylamide (PAM) |
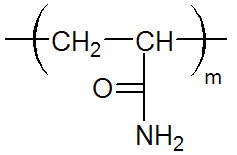
|
| Cationic Polyacrylamide (CPAM) |
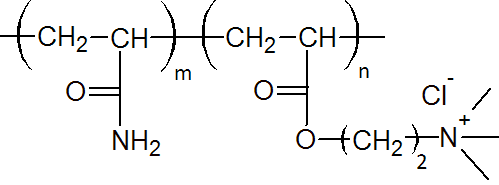
|
COMMERCIAL Polyacrylamides
Major manufacturers of acrylamide polymers are BASF, SNF Floerger, Shangdong Tongli , and Kemira.
Applications
Poly(acrylamide-co-acrylic acid) and its sodium salts (APAM) are widely used as thickening agent, binder, super absorbent, soil conditioner, filtering aid, flocculating agent, crosslinker, suspending agent, lubricant, and oil recovery agent. One of its largest uses is waste water treatment. When added to waste water, it causes suspended particles to aggregate and to precipitate. In municipal and industrial waste water treatment it can be used in all liquid-solid separation processes including primary sewage treatment. PAMs are also used to treat water from mineral mining operations. Another common use is oil extraction and recovery. When water is injected into the well, PAM assists in pushing oil locked in reservoirs towards the pump by increasing the viscosity of the injected water.
Cationic polyacrylamides (CPAM) are used for emulsion breaking, and for promoting filtration and sludge dewatering. The primary use is waste water treatment. As a primary organic coagulant, it neutralizes negatively charged colloidal particles and thus induces flocculation and sedimentation which reduces sludge volume. In paper mills, it is mainly used to enhance retention and dewatering.
Another low volume but important use of anionic and cationic polyacrylamides is gel electrophoresis for macromolecule separation. When an electic field is applied across a PAM gel, the (negatively) charged proteins or nucleic acids migrate across the gel away towards the positive electrode. Since the mobility depends on the charge and size of the molecules, each molecule species migrates differently through the gel matrix.